康 · 学术 | Reaction of the Day No. 1540
Lewis Acid-Catalyzed Direct Conversion of Carboxylic Acids into Primary Amides and Nitriles Using Bis(trimethylsilyl)amine
Shunsuke Kataoka, Kazuki Shimozono, Koki Miyazono, Hiroyuki Morimoto,* and Takashi Ohshima*
Department of Applied Chemistry, Graduate School of Engineering, Kyushu Institute of Technology, Tobata-ku, Kitakyushu 804-8550, Japan
Graduate School of Pharmaceutical Sciences, Kyushu University, Higashi-ku, Fukuoka 812-8582, Japan
—Org. Lett. 2025. doi.org/10.1021/acs.orglett.5c03348
Recommended by Shi Li_MOC
KEYWORDS: Lewis acid catalysis, amidation, dehydration (反应类型), C(sp2)-N, C(sp2)-C≡N (成键类型), Carboxylic Acids,TMS2NH(原料),amides, nitriles (产物), Yb(OTf)3, Hf(OTf)4(其他)
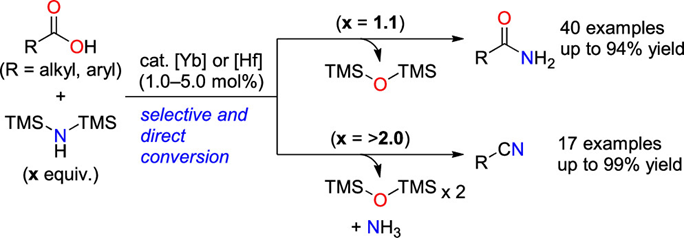
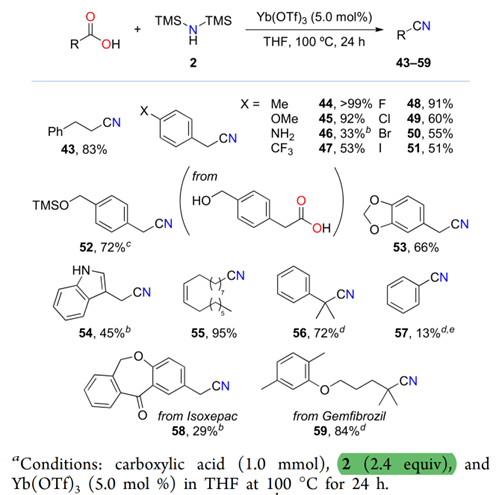
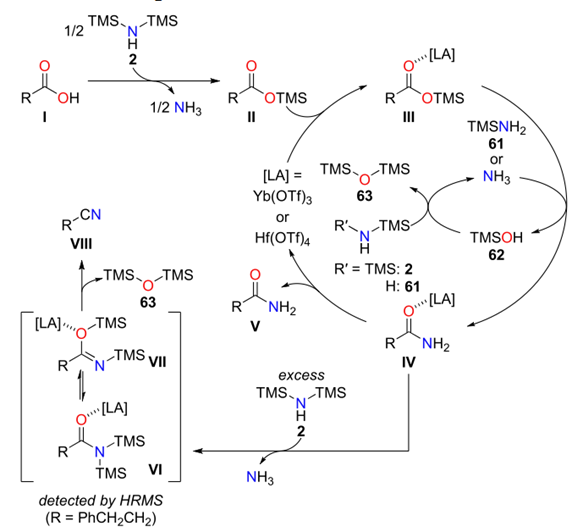
ABSTRACT: We report Lewisacid-catalyzed direct conversion of carboxylic acids into primary amides and nitriles using bis(trimethylsilyl)amine as an ammonia surrogate. With 1.1 equiv of bis(trimethylsilyl)amine, ytterbium(III) and hafnium(IV) triflates efficiently catalyzed the reaction, affording various primary amides in high yields with a broad substrate scope. Increasing the amount of bis(trimethylsilyl)amine to more than 2 equivpromoted one-pot dehydration of the primary amides to directly afford the corresponding nitriles. Preliminary mechanistic studies suggest that bis(trimethylsilyl)amine plays a key role in activating the carboxylic acids.

Introduction
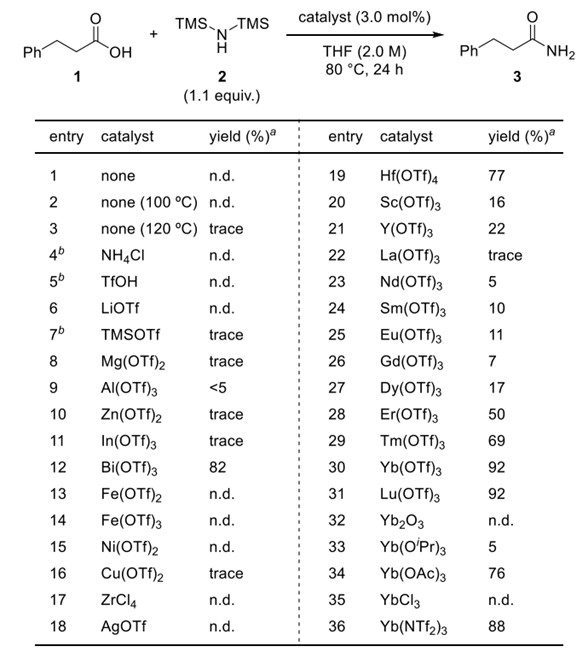
Optimization of Reaction Conditions
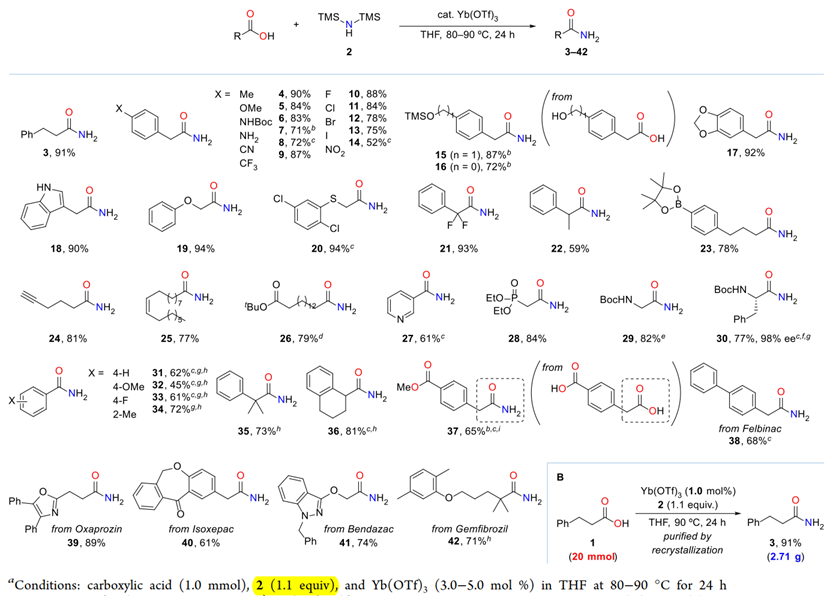
Substrate Scope of Primary Amides
Direct One-Pot Conversion of Carboxylic Acids to Nitriles


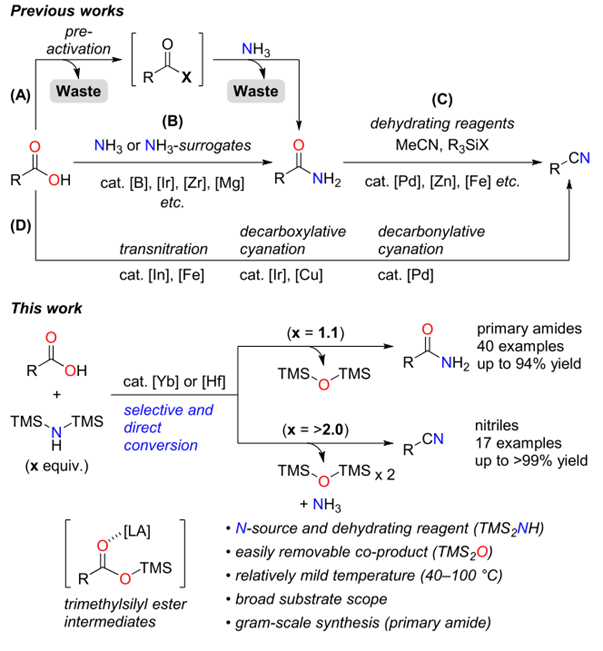
Kataoka et al. reported a Lewis acid-catalyzed direct conversion of carboxylic acids into either primary amides or nitriles using bis(trimethylsilyl)amine (TMS2NH) as an ammonia surrogate. In the presence of Yb(OTf)3, Hf(OTf)4, a wide range of aromatic, heteroaromatic, and aliphatic carboxylic acids were transformed into primary amides in high yields with broad functional group tolerance. Increasing the equivalents of TMS2NH enabled one-pot dehydration of the intermediate amides to deliver the corresponding nitriles, providing a tunable and practical protocol for selective product formation. Mechanistic studies revealed that in situ generated trimethylsilyl esters act as key intermediates and that ammonia, released from TMS2NH, functions as the actual nucleophile. The method features operational simplicity, scalability, and avoidance of toxic cyanide sources, offering a sustainable approach to access nitrogen-containing scaffolds widely relevant to pharmaceuticals and materials chemistry.